All Tree·Oss® Implants are packed inside a “ready-to-use” packaging system, thus unifying and simplifying surgical procedures.
Every Tree·Oss® implant is packed inside a double capsule system, featuring absolute safety and comfort during placement.
Case
First, you will find a case containing the blister or secondary packaging, together with registration labels and instructions for use.
- Includes registration labels and instructions for use.
- The case color indicates the type of implant.

Secondary packaging
Inside the blister or secondary packaging, you will find the primary packaging, containing the ready-to-place implant. Such packaging is a completely sterile vial with a pressure cap providing a tight seal.
Sterile vial inside an airtight blister.
- Lift-off the blister flap.
- Drop the primary packaging on the sterile field.
Primary packaging
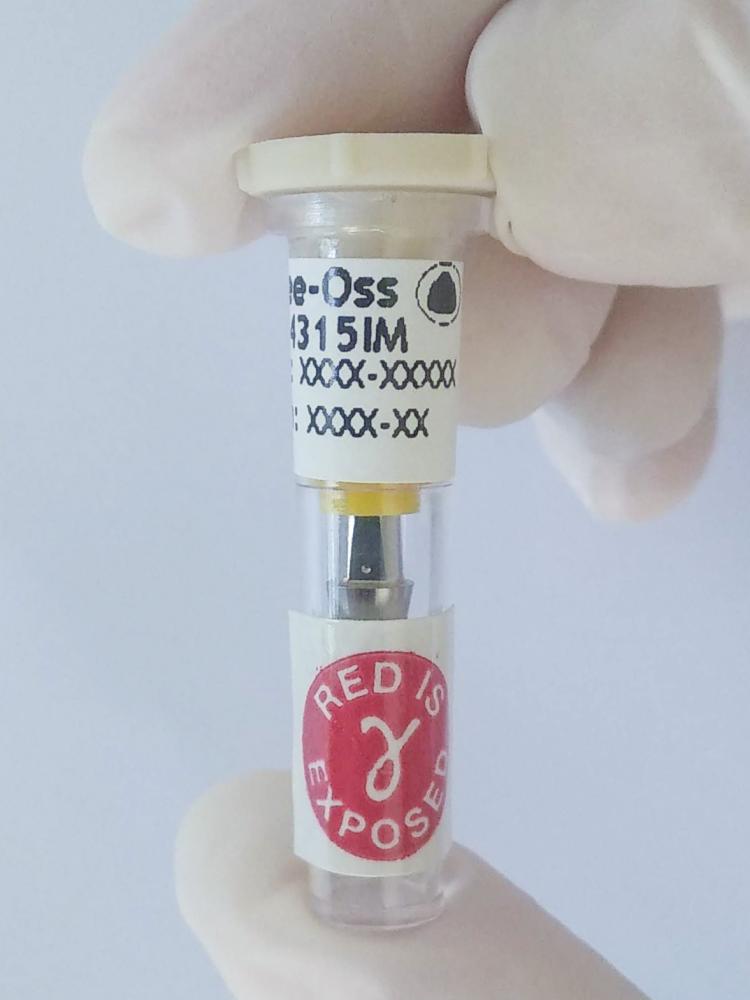
The primary packaging is sterile on the inside as well as on the outside; therefore it can be handled within the surgical field. On the other hand, due to its airtight seal, inner sterility is maintained even outside the blister.
- Sterile vial (inside and outside)
- Allows for handling within the sterile area
- Airtight seal
- Maintains sterility
- Irradiation marking. Assures gamma radiation sterilization
- Implant information. Description, size, lot number and expiration date of the product.
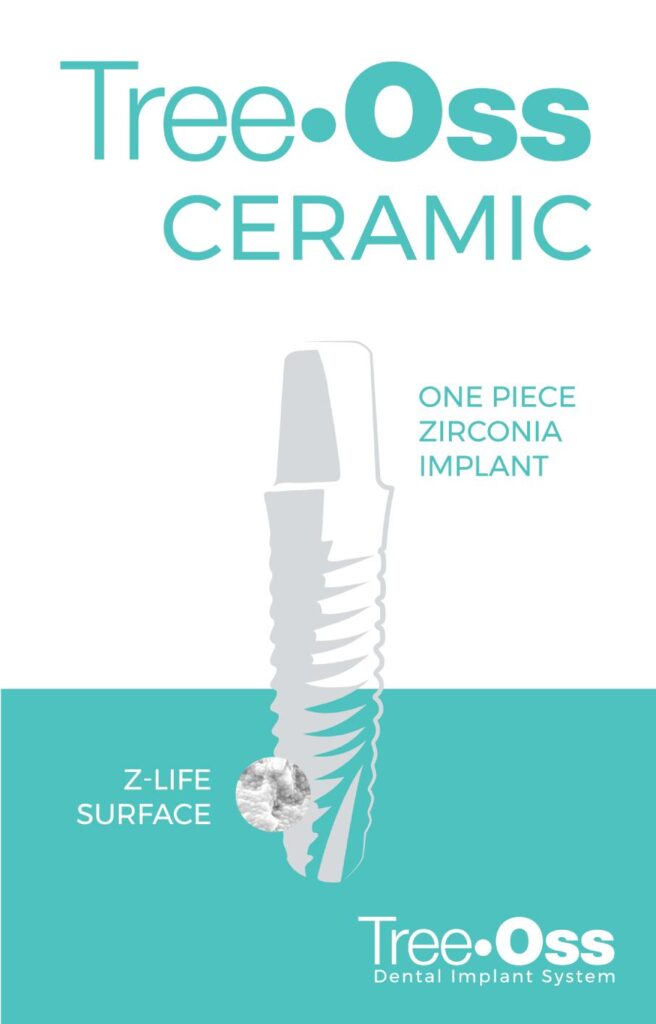
Opening and placement of Tree·Oss® Implants procedure

Use the Multifunction Implant Mount as open tray transfer or as closed tray transfer by adding a plastic SNAP.